Background:
With the increase in life expectancy, prevalence, early diagnosis of plasma cell disorders, advances in effective therapies and improved overall survival (OS) in multiple (MM); improvements in the quality standards and delivery of quality of care remain a challenge. Currently there are no standardized quality metrics and many studies have highlighted the disparities in treatment and supportive care in MM. We propose a quality model based on Donabedian's triadof structure, process and outcomes to further improve outcomes in MM. We aim to share a team process at Cleveland Clinic for defining components of quality measure for MM treatment.
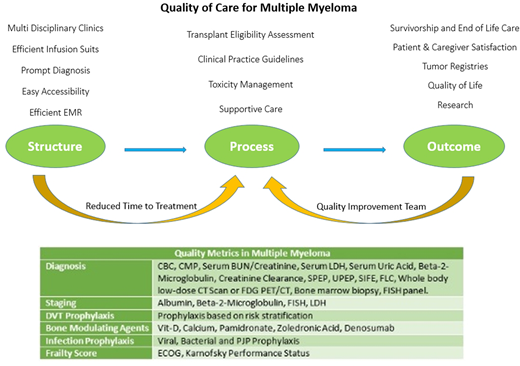
Structure:
Multidisciplinary clinics (MDCs) have become popular over the recent years and have proven to improve patient outcomes for many malignancies. Our MDC has healthcare team composed of MM physicians, palliative care physicians, nurse coordinators, clinical pharmacists, pathologists, clinical research team, basic science laboratory collaboration, and psycho-social workers. To improve efficiency, team focus is on prompt diagnosis, easy accessibility, tissue banking, efficient EMR, access to transplant /cellular therapy /immunotherapy and efficient infusion suites with focus to reduce time to treatment in the same building.
Process:
With the rapid advancements in the treatment of MM, our process is focused on the comprehensive diagnosis based on tissue analysis, comprehensive staging, risk defining genetic factor testing, and evidence based treatment selection for MM based on literature, clinical practice guidelines (CPGs) like specialty society guidelines
Recent studies have suggested that delays in treatment from diagnosis can result in negative patient outcomes, similarly studies have suggested that patients with MM can experience significant treatment delays. We focus on reduced time to treatment; recommend patients should undergo treatment within ten days of diagnosis. Patients should undergo evaluation for toxicity management, undergo supportive treatment based on CPGs. These should include: Risk estimation for DVT and score driven prophylaxis, bone modulation therapy, infection prophylaxis, management of anemia, frailty assessment and front line transplant eligibility assessment.
Outcomes:
Clinical and patient reported outcomes should be recorded, analyzed and reported. A dedicated quality improvement process should streamline data collection and select quality improvement QI projects with the aim to improve recurring issues.
Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal